SCARS2026, 16-18 January, 2026
OXARTIS, in Collaboration with MENDEDskin raised interest in ProMatrix BioDerm for skin-loss reconstruction
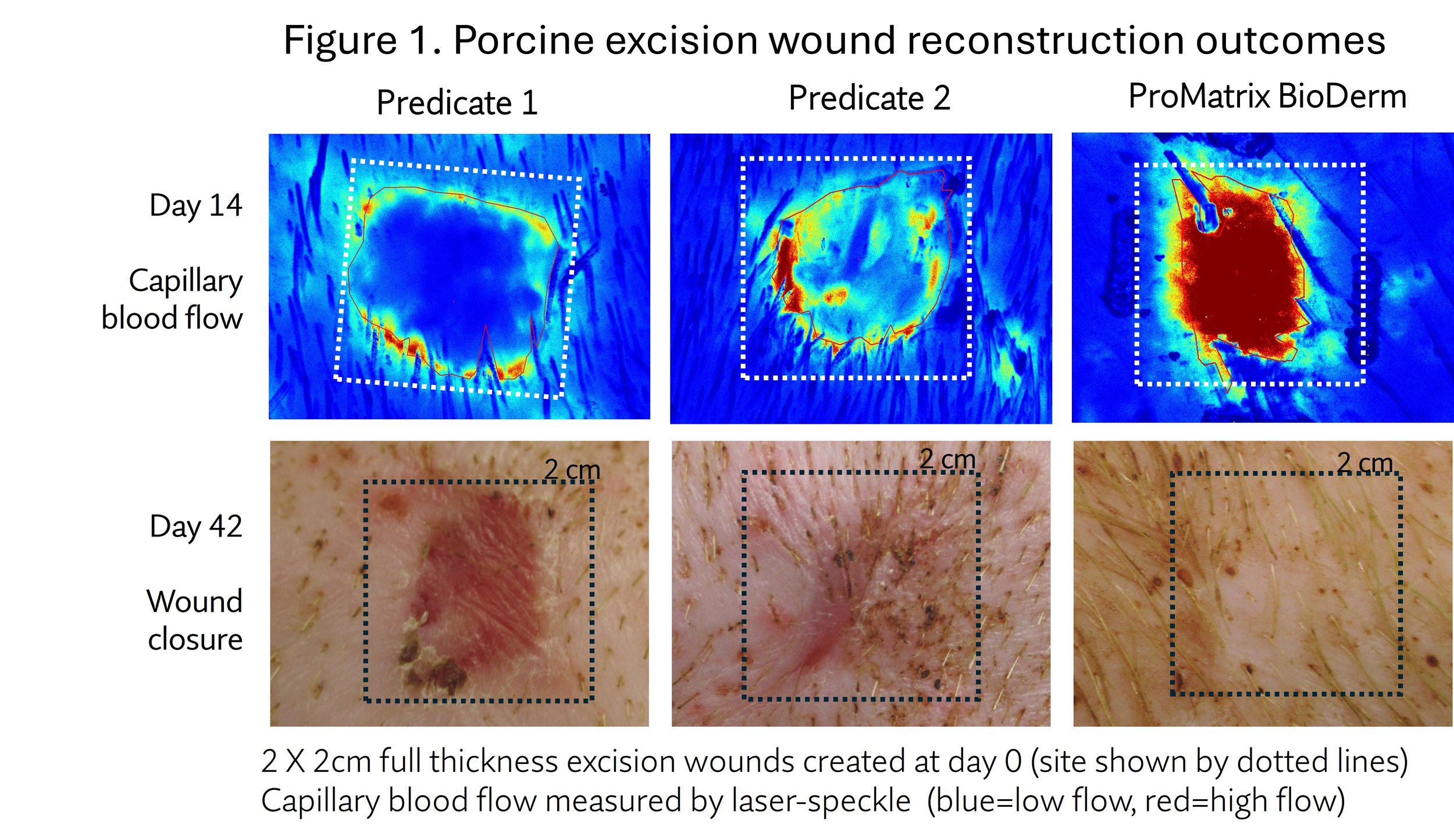
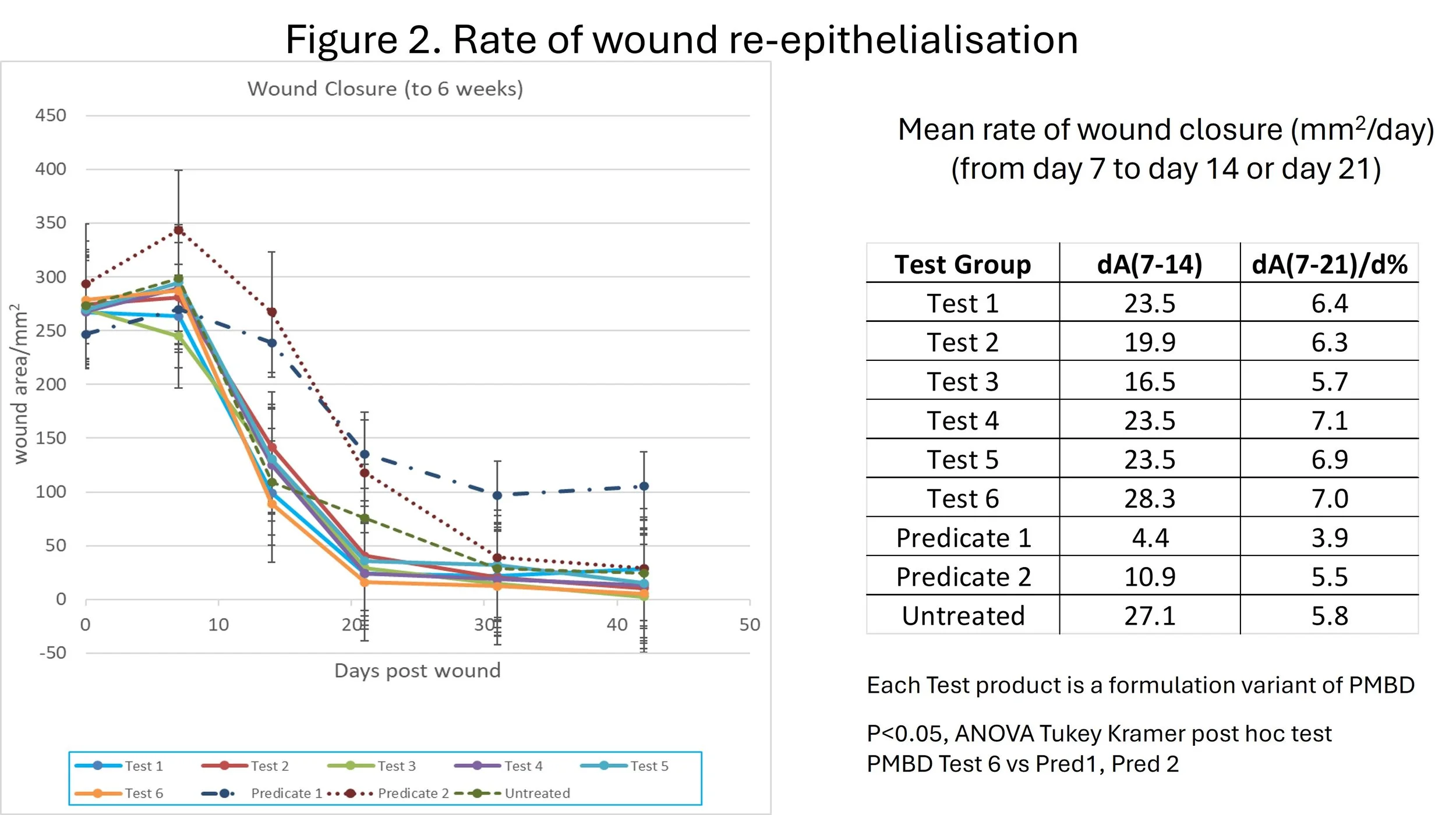
A major theme of the 6th annual conference on scars was reviewing the rapid advances in application of lasers, laser assisted drug delivery, and microdroplet transcutaneous delivery and other focal technologies for treating scars. There were some presentations on the clinical use of dermal scaffolds to reduce the extent of scarring from healing of skin-loss trauma and surgical excision wounds. However, despite reducing the extent of scarring, primary surgical outcomes of skin reconstruction typically result in dysfunctional contractures and disfigurement.
ProMatrix BioDerm scaffold technology offers a radical approach: regenerative reconstruction: prevent the wound healing process from growing scar tissue.
ProMatrix would not obviate the use of treatments for existing scars, but stands to accelerate recovery and rehabilitation from the most severe wounds.
We are very grateful for great interest of the delegates, especially burns and reconstructive surgeons, and we look forward to progressing the essential regulatory evidence needed for clinical use.